Online Interactive Workshop
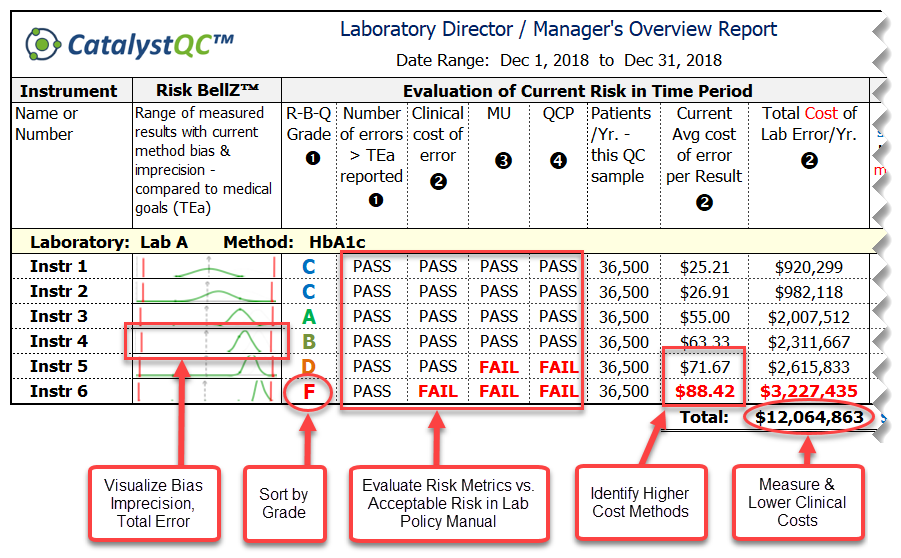
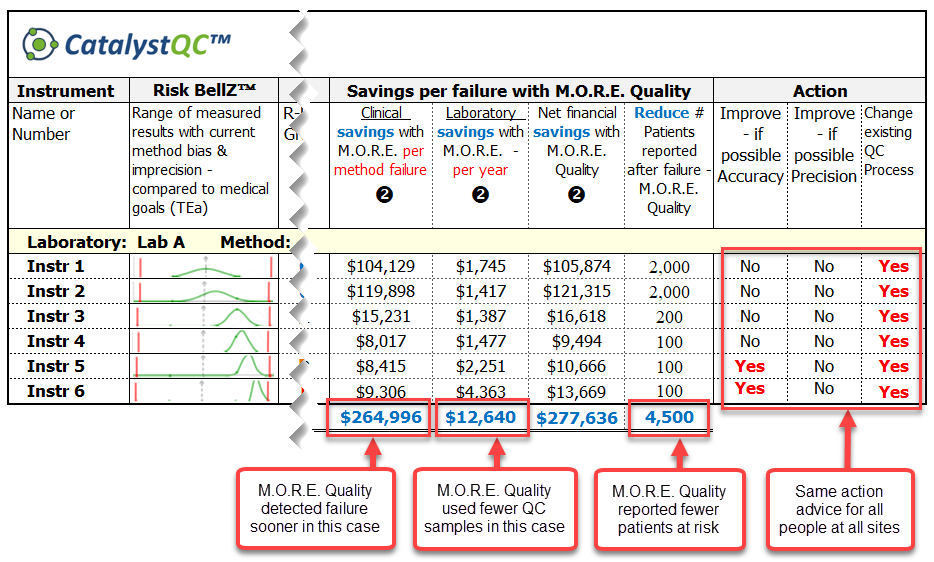
Use NEXT GENERATION technology in medical laboratory quality/risk management to project clinical savings throughout the health care system - a test case to study HbA1c.

Premiere
Special!
What are the clinical costs and risks to patients and healthcare systems of errors in HbA1c results that mislead doctors to either:
[A] mis-classify patient as low risk instead of high risk of diabetes, or
[B] think that patients are improving or not improving when the opposite is actually true?
Validate the effectiveness of existing quality control processes to detect medically-significant error. Prepare to be shocked by this realtime demo!
Regular Multi-Lab Workshop Price $497.00 Introductory sale $124.25
Use NEXT GENERATION technology in medical laboratory quality/risk management to project clinical savings throughout the health care system - a test case to study HbA1c.
Sign up now for the chance to submit your data!